This article is available in several languages:

Fire protection matters: which gas to use?

Does the sprinkling of CO2 actually make a difference to an inert gas blend, or is it just marketing magic?
-
Automatic (clean gaseous) fire extinguishing systems: inert gases – which, blend or not, and why?
The benefits and consequences of a blend containing CO2
-
Executive Summary
Many Original Equipment Manufacturers’ (OEM’s) fixed automatic fire extinguishing portfolios includes inert gases, in their pure form or as blends. Since the 1990s, Inergen® remains the generic name for inert gas extinguishing systems perhaps as Hoover® is to vacuum cleaners.
This paper identifies what the benefits are of having an inert gas a blend containing carbon dioxide (CO2), such as IG-541 which was brought to the market under the product name Inergen®.
In a non-fire discharge such blends containing CO2 arguably lower blood-oxygen quicker, and in a fire situation they increase the uptake of noxious and potentially toxic smoke as well as any other by-products of combustion.
The conclusion is that a blend containing CO2 could be regarded as disadvantaged when compared against the pure inert gases, having also identified some of the commercial benefits and ramifications of using a blend.
Irrespective, the need for automated fire protection is increasing as is our expectation of uptime and digital availability. A system that can avert or extinguish a fire and allow a business to continue to operate, whilst counter-measures to address the situation are deployed, is paramount and it is here that inert gases are ideally suited, now matter what the blend. Proper design, installation and ongoing maintenance is however fundamental to success.
-
Introduction
Questions have been raised as to the benefits of an inert gas (or blend), in addition to the pure gases on the market, with the addition of carbon dioxide.
-
Inert Gas Blends Containing Carbon Dioxide And Their Effect On Respiration
- Atmospheres containing carbon dioxide (CO2) concentrations of around 3% by volume increase the rate of respiration in humans.
- IG-541 is the only recognised inert gas blend used for fire protection with the addition of CO2. This is presented to the market as having the advantage in low oxygen atmospheres of increasing the rate of respiration and therefore the uptake of oxygen.
- The New Extinguishants Advisory Group (NEAG), a sub-group of the United Kingdom Halon Alternatives Group (HAG) identified the negatives of such increased respiration as “the induced increase in ventilation and possible reduction in breath-hold time may enhance the uptake by the body of toxic materials from the fire.”
-
Physiology Of Respiration
The human body is a biological engine in which the lungs act as both an air intake and exhaust. When air is drawn into the lungs, oxygen passes from the alveoli (the air sacs within the lungs where gas transfer occurs) into the bloodstream and carbon dioxide passes from the bloodstream into the alveoli. This process of gas transfer is known as diffusion.
Diffusion operates on the principle that a gas will move from an area of high concentration to one of low concentration until equilibrium is established (i.e. the concentration is equal in both areas). When air is drawn into the lungs the concentration of oxygen in the air is higher within alveoli than in the capillaries that flow around them; oxygen then passes from the alveoli into the blood. Conversely, the concentration of carbon dioxide in the blood is higher than in the air; carbon dioxide then passes from the blood into the alveoli. During exhalation the carbon dioxide is then expelled.
The amount of oxygen carried by the blood depends primarily upon the concentration of oxygen within the inhaled air. This is shown by the graph.
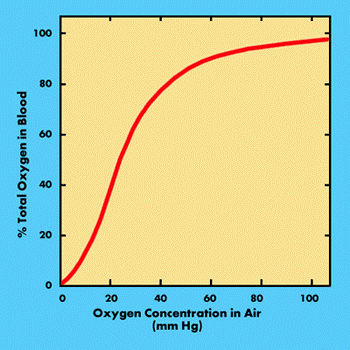
As the atmospheric concentration of oxygen decreases, the amount carried by the blood initially shows only a gradual decrease. When the atmospheric oxygen concentration is 10.5 % (i.e. 50 % of normal), the blood is still carrying approximately 85 % of its normal level.
It is only when the atmospheric concentration falls below 8.4 % that there is a marked decrease in the amount of oxygen carried (Hammer, 1988; Timar, 1983). Most healthy humans, at the expense of increased demands upon the cardiovascular system, can typically tolerate oxygen concentrations down to approximately 12 %. An oxygen concentration of 15 % for example, will show no immediate effects in most people, but the load upon the cardiovascular system is equivalent to a workload of 3 kcal / min (Timar, 1983).
The blood normally holds reserves of oxygen. The blood returning to the lungs (to release carbon dioxide) still carries over 40 % of the oxygen it held initially. This is why you can hold your breath for some minutes without adverse effects. Your body is able to consume this “reserve store” of oxygen. However, if you were to continue to breathe in an atmosphere containing very low levels of oxygen (where the air drawn into the alveoli has a lower concentration of oxygen than in the blood flowing around them), the diffusion process would operate in reverse. With every breath you take, you would be expelling oxygen instead of absorbing it.
-
Increased Respiration
Considering the above, the CO2 in IG-541 will prompt increased respiration. This conceivably can accelerate reduction the residual oxygen contained within the blood, via reversed diffusion, at a greater rate than would be the case with any inert gas that does not contain CO2.
-
Argon And Nitrogen Inert Gases
Originally the UK adopted two pure inert gases to the market; argon (IG-01) and nitrogen (IG-100). Many fire extinguishing systems are available utilising either gas and carried credible third party attestation.
Each gas has its own merits under specific circumstances, and these should be evaluated when appraising any risk requiring protection. Critical factors include:
- The fuel type; some fuels (magnesium or radioactive risks for example) require specific extinguishants.
- Certain fuel types may require significantly higher levels of one extinguishant than the other.
- The specific gravity of nitrogen being 0.967 in air (argon being 1.38) may make it more suitable for use in an area having larger unclosable openings (subject to where they are).
- The geometry of the enclosure and the height of the protected risk / asset within it. Nitrogen should provide full height coverage during the retention period without the need for mechanical mixing.
- The location of leakage paths which can not be addressed / sealed.
Considering a blend, and the addition of CO2 to this blend, this may offer no appreciable fire-fighting or agent retention (hold-time) benefits over the two pure gases that are time proven and already available.
-
Why Blend Inert Gases
OEM seeking a unique proposition created blends, with trade names such as Inergen® IG-541 and Argonite™ IG-55. This enabled them to have their proprietary product specified for an opportunity.
Users and specifiers may have sought solace in the fact their system was to come from a ‘credible’ supplier, or as a means to avoid suppliers of generic solution, some of whom may not have necessary approvals or product lifetime certainty.
Certainly we saw such OEMs invest heavily in proving the efficacy of their proprietary blend, and this may have provided competitive advantage; where less gas is required the marginal cost of fewer cylinders can be appreciable. Conversely chemists may dispute this improved efficacy; expense proved the knife-edge concentration after which an agent would fail to extinguish a fire, so not actually more efficient, just a lesser unknown safety factor.
-
Summary
- In a non-fire situation, there is no benefit to the CO2 being present in IG-541; there is sufficient oxygen within the air for people to breathe.
- Elevating the atmospheric CO2 will prompt increased respiration which will reduce the residual oxygen contained within the blood, via reversed diffusion, at a greater rate than an inert gas without CO2.
- In a fire situation the increased respiration, brought about by the presence of CO2, will increase the human uptake of noxious and potentially toxic smoke, as well as any other by-products of combustion. These may be more likely to result in injury than the fire itself. Inhalation of the products of combustion may also lead to more rapid incapacitation, which negates the claimed benefit of Inergen®.
- Systems within the UK employ coincidence (often termed – correctly or not – “double-knock”) detection and a pre-discharge delay to allow persons to escape. Even following a discharge, all well-designed and maintained inert gas extinguishants should pose no appreciable threat to the safety of anyone incapacitated or trapped within the protected area.
- A patented blend can restrict a customer’s choice as to who can maintain and refill the system. Many clients strongly oppose such lifetime tie-ins.
- Blends can delay the refilling of systems following a discharge, limit the availability of those capable of refilling the systems and add to the cost of refilling.
-
Conclusion
Although well marketed, IG-541 and blends with CO2 could be regarded as less desirable when compared to pure noble inert gases.
Without doubt, persons should be warned of an imminent discharge and afforded time to evacuate.
Similarly, systems should be designed so they need not be incapacitated whilst persons are present, to avoid fallibility of being left isolated, etc.
A credible maintainer then routinely attests the system’s concentration remains acceptable for the volume and fire risk, and any considers the threat to life safety.
Do I believe inert gas fire extinguishing systems are any good? Without doubt, yes!
Inert gas systems are clean, and given the increasing dependence upon system (IT) availability, there is no shadow of doubt they are ideal for protecting critical assets and irreplaceable artefacts especially where continued operation is imperative.
-
References
- Report By The Halon Alternatives Group (HAG). “A Review Of The Toxic And Asphyxiating Hazards Of Clean Agent Replacements For Halon 1301.” Originally produced in February 1995, revised February 2000 and May 2001.
- The Safety-Line Institute of Work-Safe Western Australia.
- Timar, M. “Hypoxia and Anoxia”. In Parmeggiani, L. (Ed.), Encyclopaedia of Occupational Health and Safety. ILO Geneva, 1983 (3rd Ed.).
- Hammer, W. Occupational Safety Management and Engineering. Prentice-Hall, New Jersey, 1981.
Join the conversation!